
Electrons are the smallest of these particles and travel in shells or orbitals around the nucleus, sometimes called the electron cloud. The nucleus makes up more than 99.9% of an atom’s mass. Neutrons have no charge and are also located in the nucleus. The number of protons is the element’s atomic number. Each element has a different number of protons. The number of protons determines which element the atom represents. Protons are positively charged and located in the nucleus, the center of the atom. Everything that you see, smell, hear, taste, and touch consists of atoms.Ītoms are made up of three sub-atomic particles called protons, neutrons, and electrons. Since there are over 110 elements, there are over 110 types of atoms. An element is matter that is made up of one type of atom. Is the smallest part of a chemical element that retains the properties of that element. The difference of these measurements is the volume of the object.Īn atom is the basic unit of matter. The amount of water in a graduated cylinder can be noted before an object is placed in it and after the object is placed in it. To measure an irregularly-shaped object, water displacement can be determined using, for example, a graduated cylinder (assuming the object has a higher density than water). Volume can be measured using a meter stick to measure the sides of a regularly-shaped object. It can be measured in cubic millimeters or cubic centimeters. Volume is three-dimensional and incorporates length, width, and height. For instance, a volley ball has a larger volume than a golf ball because it takes up more space. An object with a lot of mass is more difficult to put in motion or stop if already in motion compared to an object with a small amount of mass. The more massive an object is, the more inertia it has. Furthermore, mass has a positive correlation with inertia. Mass does not take into consideration gravity and is constant. Gravity is different on different celestial bodies, so weight changes according to where it is measured. This is different than using a scale to determine weight because the reading on a scale for a particular object depends on the force of gravity.

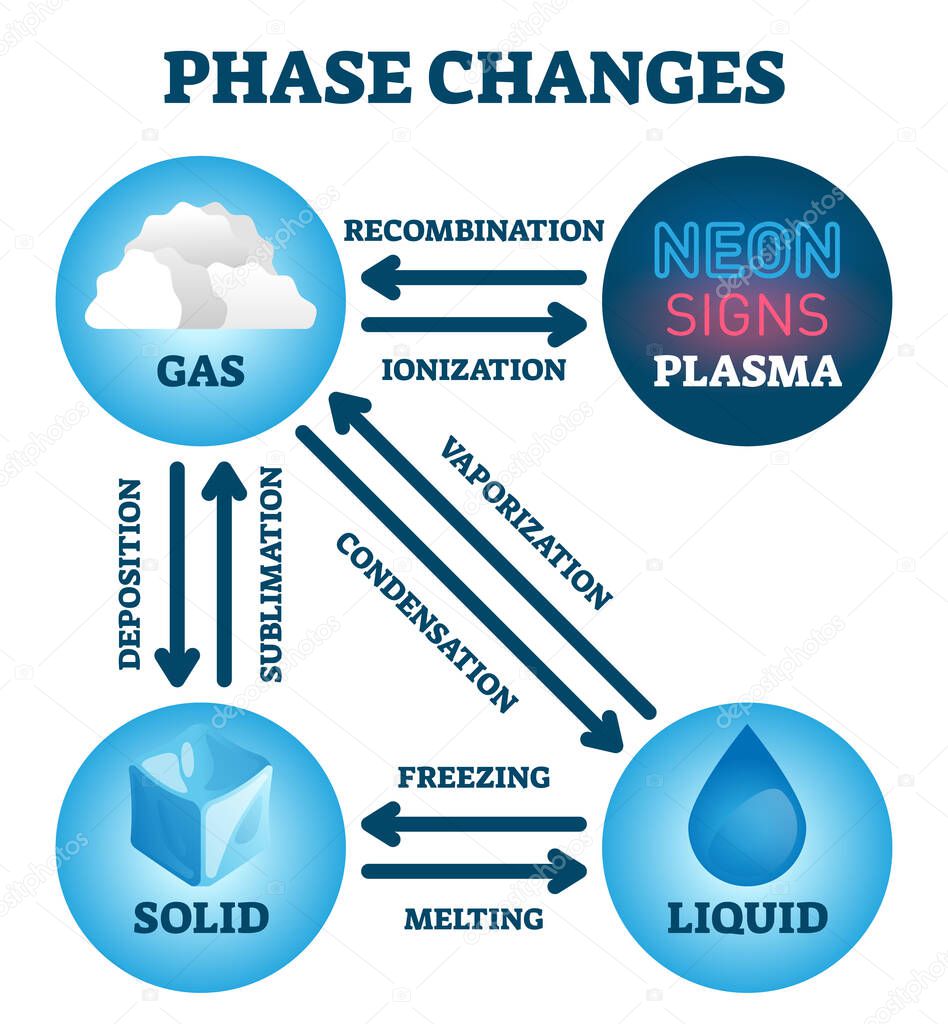
It can be measured in milligrams, grams, or kilograms using an analytical electronic balance or a triple beam balance. For instance, a cake has more matter that a slice of that same cake. Light energy, sound energy, heat energy, potential energy, and kinetic energy are examples. Energy is the capacity to cause change and is not matter. If it can be proven that a substance has mass and takes up space, it is matter. There are six ways a substance can change between these three phases melting, freezing, evaporating, condensing, sublimination, and deposition (2).Matter is any substance that has mass and volume. In the example of ice melting, while the ice is melting, there is both solid water and liquid water in the cup. This typically happens when the substance is transitioning from one phase to another. There can be two phases coexisting in a single container at the same time. The temperature and pressure at which the substance will change is very dependent on the intermolecular forces that are acting on the molecules and atoms of the substance (2). Every substance is in one of these three phases at certain temperatures. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure.Įach substance has three phases it can change into solid, liquid, or gas (1). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state.


 0 kommentar(er)
0 kommentar(er)
